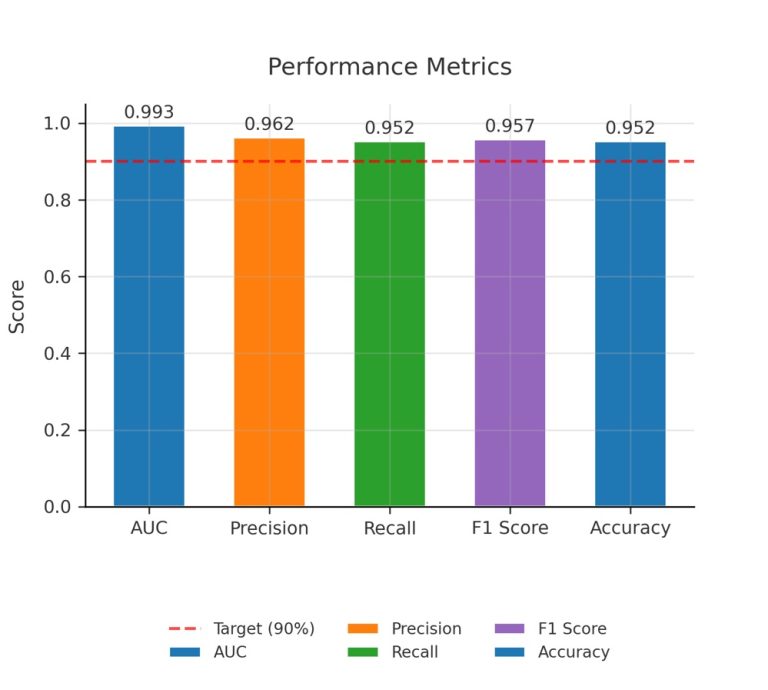
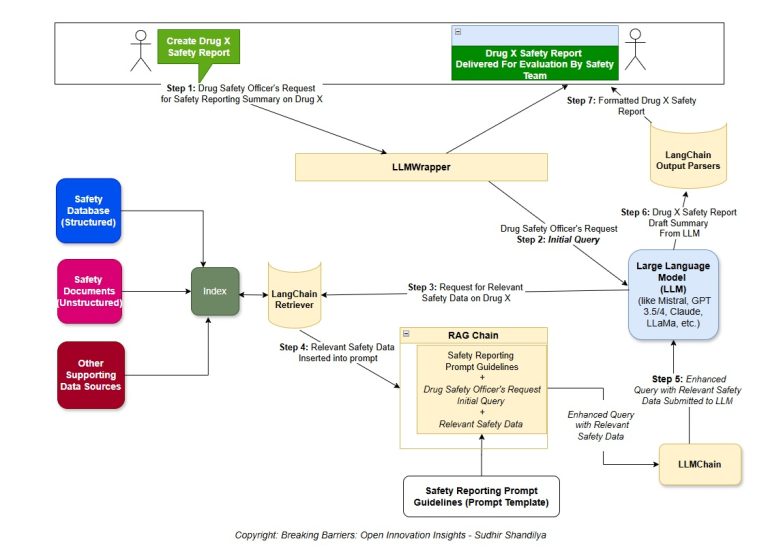
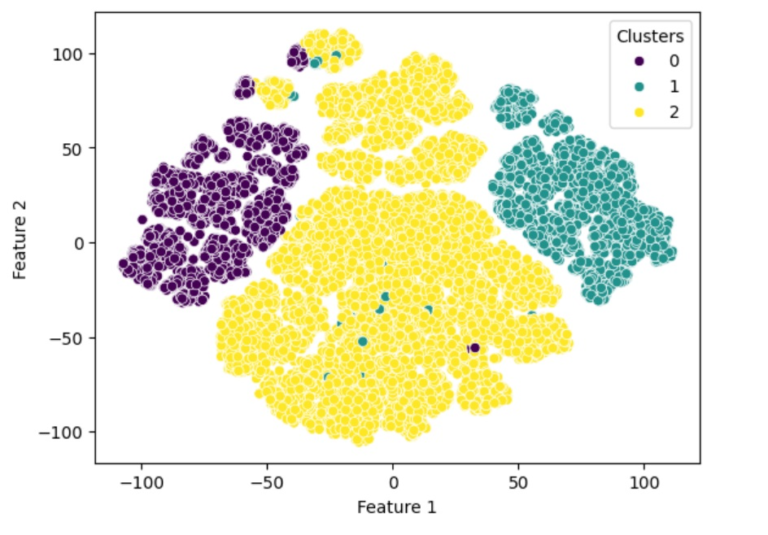
Designing a system to ensure that AI technology behaves ethically, follows the rules, and acts responsibly when handling data is complex. This system should include human thinking and behavior elements to ensure that the AI makes decisions in line with what humans consider ethical. Below, is a suggested plan along with a comparison to how humans think, and some examples related to clinical trials:
Framework for Ethical and Compliant AI Solutions:
Establish Clear Ethical Guidelines:
-
- AI: Develop ethical guidelines that the AI must adhere to, focusing on fairness, non-discrimination, transparency, and accountability.
-
- Human Comparison: Humans process ethical decisions based on societal norms, personal values, and empathy. The AI’s ethical guidelines should mirror these human considerations.
-
- Clinical Trials Application: Ensure that the AI system used in clinical trials does not discriminate based on race, gender, or other irrelevant factors when selecting participants or analyzing data.
Ensure Regulatory Compliance:
-
- AI: Build compliance checkpoints into the AI development process to ensure adherence to all relevant laws and regulations, such as GDPR for data privacy or HIPAA for healthcare information.
-
- Human Comparison: Just as professionals adhere to industry regulations and legal requirements, AI systems must have built-in mechanisms to ensure compliance.
-
- Clinical Trials Application: Implement AI systems programmed to comply with clinical trial regulations, such as informed consent protocols and reporting adverse events.
Implement Data Privacy Measures:
-
- AI: Use data anonymization, encryption, and secure data storage to protect personal information.
-
- Human Comparison: Humans have an inherent understanding of privacy. AI systems must be designed to respect and protect data privacy like humans would handle confidential information.
-
- Clinical Trials Application: Ensure that patient data used by AI in clinical trials is anonymized and securely stored, maintaining patient confidentiality.
Conduct Regular AI Ethics Audits:
-
- AI: Periodically audit the AI system to adhere to ethical guidelines and regulatory requirements.
-
- Human Comparison: Humans reflect on their actions and decisions to ensure they align with ethical standards. AI systems require similar ongoing evaluations.
-
- Clinical Trials Application: Regularly audit AI systems used in clinical trials for ethical decision-making, particularly in patient selection and data analysis.
Facilitate Human Oversight:
-
- AI: Design the AI system for human intervention and oversight, especially in critical decision-making processes.
-
- Human Comparison: Humans exercise judgment and can intervene when necessary. AI should not operate in isolation and must be subject to human oversight.
-
- Clinical Trials Application: Enable clinicians to oversee and moderate AI-driven processes, such as patient diagnosis or treatment recommendations.
Promote Transparency and Explainability:
-
- AI: Ensure the AI system’s decision-making process is transparent and its actions can be explained in understandable terms.
-
- Human Comparison: Humans can explain their reasoning and decisions. Similarly, AI should define its activities, particularly in sensitive areas like healthcare.
-
- Clinical Trials Application: Implement AI systems that can explain their reasoning for patient selection or data analysis outcomes in clinical trials.
Incorporate Diverse Perspectives:
-
- AI: Involve diverse stakeholders in developing and monitoring the AI system to ensure it considers various perspectives.
-
- Human Comparison: Human decision-making benefits from diverse viewpoints. AI should be developed with input from multiple perspectives to avoid biases.
-
- Clinical Trials Application: Engage diverse stakeholders, including patients, clinicians, and ethicists, in developing and monitoring AI systems used in clinical trials.
Actual Application in Clinical Trials:
Imagine an AI system designed to assist in patient recruitment for clinical trials. This system uses algorithms to analyze patient data and identify potential candidates. To ensure ethical and compliant operation, the system:
-
- Adheres to established ethical guidelines, ensuring fairness in patient selection.
-
- Complies with clinical trial regulations and data privacy laws.
-
- Uses anonymized patient data and has security measures to protect this data.
-
- It is regularly audited for ethical compliance and adherence to regulations.
-
- Allows clinicians to review and override AI recommendations.
-
- Provides transparent and understandable reasons for selecting or excluding patients.
-
- It is developed and monitored with input from a diverse group of stakeholders.
By incorporating aspects of human cognitive behavior and ethical processing, this AI framework ensures that the system operates ethically, complies with regulations, follows responsible AI practices, and upholds data privacy standards, particularly in the sensitive context of clinical trials.